The flue gas desulfurization pH electrode adopts a flat glass ball, which has good anti-pollution and impact resistance. The electrode pH probe has a faster reaction rate, accurate measurement, and a long-life reference system to improve the stability of the reference electrode.

Upper and lower 3/4NPT pipe threads
- Easy installation, no sheathing required for conventional testing.
- Teflon reference fluid, high and low-temperature resistance, acid, and alkali resistance Non-adhesive.

New Design Glass Bulb
- Prevents interference bubbles in the internal buffer.
- Fast response, accurate measurement, and good stability.
- No hydrolysis, reliable measurement.
Application
pH Electrode is widely used in the continuous testing and monitoring of pH, ORP and temperature in thermal power, chemical fertilizer, metallurgy, environmental protection, pharmaceutical, biochemical, food, drinking water, etc.

| Model | SISCO-PHETE-PH5012 |
| Weight | 1kg |
| Measuring range | 0-14pH |
| Accuracy | 0.02pH |
| Temperature range | 0-60℃ (32-140℉) |
| Temperature compensation * | Internal temperature sensor (NTC 10K) |
| Zero position | 7±0.3pH |
| Compressive strength | 0.6MPa |
| Slope | ≥96% |
| Installation size | 3/4NPT in both ends |
| Shell material | PPS/PC |
| Connection | Direct lead |
| Cable length * | 5m/10m/15m, or other length less than 50m (need customization) |
| Service life | 6 months |
Installation
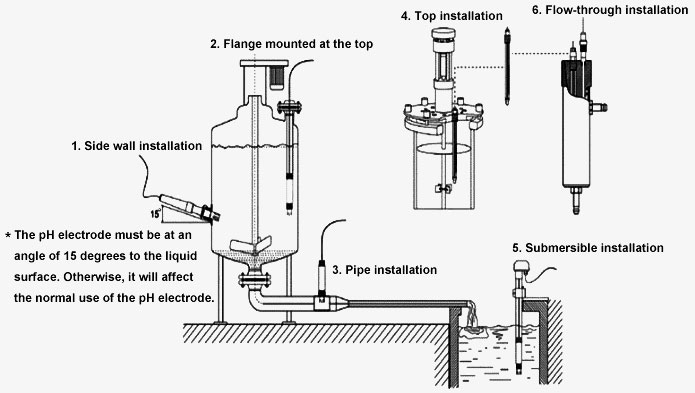
- Side wall installation (*The pH electrode must be at an angle of 15 degrees to the liquid surface. Otherwise, it will affect the normal use of the pH electrode.)
- The flange mounted at the top
- Pipe installation
- Top installation
- Submersible installation
- Flow-through installation
Dimension (unit: mm)

Q1: What is a pH electrode?
A1: The pH electrode is also called the pH probe pH sensor. The pH electrode is the part of the pH meter that is in contact with the substance to be measured and is used to measure the electrode potential.
Q2: Why do pH electrodes need to be soaked?
A2: The pH electrode must be soaked before use, because the pH bulb is a special glass membrane with a thin hydrated gel layer on the surface of the glass
membrane, which can only interact with the H+ ions in the solution under fully wet conditions. the response to. At the same time, after soaking the glass
electrode, the asymmetric potential can be greatly reduced and tend to be stable.
Q3: What is a reference electrode?
A3: An electrode with a known and constant electrode potential that does not respond to the activity of hydrogen ions in solution is called the reference electrode. Reference electrodes include mercurous sulfate electrodes, calomel electrodes, and silver/silver chloride electrodes. The most commonly used are calomel electrodes and silver/silver chloride electrodes. The role of the reference electrode in the measurement cell is to provide and maintain a fixed reference potential, so the requirements for the reference electrode are that the potential is stable and reproducible, the temperature coefficient is small, and the polarization potential is small when current flows.
Tips: What is a two-junction reference electrode?
The double junction reference electrode consists of two junction boundaries and two reference solution chambers. The outer chamber is generally filled with potassium nitrate solution, while the inner chamber is filled with potassium chloride solution. It has two distinct advantages. One is to reduce pollution. The tested solution can only contaminate the solution in the outer chamber and has limited influence on the kcl solution in the inner chamber. Second, it reduces the clogging of the liquid junction and chemical reactions between ions.
Thank you for buying industrial test and measurement equipment on SISCO.com, all products sold by SISCO and the partner cover a 12 months warranty, effective from the date of receiving the products.
What is covered?
SISCO is responsible for providing free spare parts, and free technical support to assist the customer to repair the defective products until the problem is solved.
What is not covered?
- Product purchased from anyone other than a SISCO store or a SISCO authorized reseller.
- Expendable parts.
- Routine cleaning or normal cosmetic and mechanical wear.
- Damage from misuse, abuse or neglect.
- Damage from use of parts other than SISCO approved.
- Damage from use outside the product’s usage or storage parameters.
- Damage from use of parts not sold by SISCO.
- Damage from modification or incorporation into other products.
- Damage from repair or replacement of warranted parts by a service provider other than a SISCO authorized service provider.
- Damage caused by the application environment not meeting the product usage requirements and the failure to perform preventive maintenance.

