The digital pH meter is output to the monitoring room for record preservation through RS485 or current transmission, and historical data can be viewed through the record option. The sisco meter is widely used in thermal power, chemical fertilizer, metallurgy, environmental protection, pharmaceutical, biochemical, food, and water industries.
Watchdog protection system, strong anti-interference ability
- 2.4 inch LCD display.
- Good anti-interference performance.
- Manual/automatic temperature compensation.
- User settings: Language, buzzer, LCD backlight, password, pH/ORP.
- electrode, manual/automatic temperature compensation, pH/ORP calibration, pH/ORP/temperature modification, pH/ORP high/low alarm, output signal 4-20mA and RS485, etc.
- Internal industrial watchdog insures pH meter doesn't crash.
Application
Digital pH/ORP meter is widely used in the continuous testing and monitoring of pH, ORP and temperature in thermal power, chemical fertilizer, metallurgy, environmental protection, pharmaceutical, biochemical, food, drinking water, etc.

| Model | SISCO-PHCR-PH160 |
| Weight | 1kg |
| Dimension | 96*96*100mm (H*W*D) with hole size 92*92mm |
| Measuring range | pH: 0~14pH, ORP: -1000~1000mV (-2000~2000mV can be customizable) |
| Accuracy | ±0.02pH, ±1mV |
| Resolution | ±0.01pH, ±1mV |
| Stability | ≤0.02pH/24h, ≤3mV/24h |
| Input impedance | ≥10^12Ω |
| Temperature range | 0~100℃ (NTC 10K, accuracy: ±0.5℃) |
| Temperature compensation | 0~100℃ manual/automatic |
| Output signal | Isolated 4-20mA signal (error: ±0.2%, under maximum loop resistance 750Ω) |
| Communication function | RS485 with standard MODBUS-RTU (Now RS485 software is free.) |
| Power supply | AC 220V±10%, 50Hz/60Hz DC 24V |
| Alarm relay | 2 alarm relays: AC 250V/3A Function: high alarm, low alarm |
The pH meter and pH/ORP electrode selection
| pH meter | pH/ORP electrode | Industry | Temperature range | Advantage |
| SISCO-PHCR-PH160 | Combination pH electrode | Sewage disposal | 0-60℃ (32-140℉) | Combination electrode has automatic temperature compensation function, suitable for occasions with large temperature fluctuations. |
| Pure water pH electrode | Drinking water | 0-60℃ (32-140℉) | The pH calomel electrode can be used in strong acid or alkali. | |
| PTFE pH electrode | Chemical | 0-60℃ (32-140℉) | The electrode shell is made of PTFE, suitable for corrosive solution (strong acid or alkali), has longer life in harsh environments. | |
| pH glass electrode | Sewage disposal/food | 0-95℃ (32-203℉) | Direct lead, double-layer glasses. | |
| Desulfurization pH electrode | Desulfurization | 0-60℃ (32-140℉) | Flat electrode, strong anti-pollution ability and longer life | |
| High temperature/sterilization pH electrode | Food | -5℃-130℃ (23-266℉) | The electrode can withstand high temperature of 0-130℃, suitable for occasions of high temperature sterilization in pharmaceutical factories, breweries, etc. | |
| High Pressure pH electrode | Laboratory | -5℃-130℃ (23-266℉) | Its linearity and accuracy are higher than all above electrodes. |
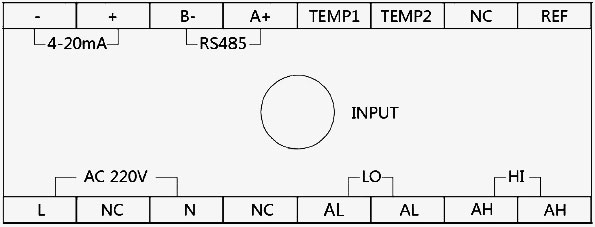
The pH/ORP meter diagram of ports definition
Communication
RS485 communication data and register address as follows:
| Address | Data type | Function code | Explanation | Access right |
| 0x0000 | unsigned short | 0x03 | pH value (default in two decimals) | Read only |
| 0x0001 | unsigned short | 0x03 | Temperature value (default in one decimals) | Read only |
| 0x0002 | short | 0x03 | ORP value (signed integer) | Read only |
Q1: What is the working principle of a pH meter?
A1: The pH meter works on the principle of a galvanic battery. The electromotive force between the two electrodes of the galvanic battery is based on Nernst's law, which is not only related to the properties of the electrodes, but also to the concentration of hydrogen ions in the solution. There is a the corresponding relationship between the electromotive force of the primary cell and the hydrogen ion concentration, and the negative logarithm of the hydrogen ion concentration is the pH value.
Q2: The same sample is measured on two pH meters at the same time, why are the readings inconsistent?
A2: Because the calibration conditions of the two pH meters are different (such as calibration done at different times), the measured values are different. Therefore, the pH meter should be calibrated at the same time with the same buffer, and then measured at the same time.
Q3: How do I know if the pH meter is accurate?
A3: Take three standard buffer solutions: pH 6.86, pH 4.00, pH 9.18 (preferably freshly prepared and at the same temperature), position calibration at pH 6.86, slope calibration at pH 4.00, and then test pH 9.18 to know the pH meter Is it accurate.
Tips: What is 1 point calibration of the pH meter?
Any kind of pH meter must be calibrated by pH standard solution before measuring the pH value of samples. For samples with measurement accuracy below 0.1pH, one point calibration method can be used to adjust the instrument, usually pH 6.86 or pH 7.00 standard buffer solution is used. Some instruments only have 0.2pH or 0.1pH, so the instrument only has a positioning adjustment twist, the specific operation steps are as follows.
- Measure the temperature of the standard buffer solution, check the table to determine the pH value at that temperature, and adjust the temperature-tasting knob to that temperature.
- Rinse the electrode with pure water and shake it dry.
- Immerse the electrode in the buffer solution and shake it to rest. After the reading is stable, adjust the positioning knob to make the instrument display the pH value of the standard solution.
- Remove the electrode, rinse and shake dry.
- Measure the temperature of the sample and adjust the temperature compensation knob of the pH meter to that temperature value.
- Immerse the electrode into the sample solution, shake it and leave it at rest, then read the value when the display is stable
Thank you for buying industrial test and measurement equipment on SISCO.com, all products sold by SISCO and the partner cover a 12 months warranty, effective from the date of receiving the products.
What is covered?
SISCO is responsible for providing free spare parts, and free technical support to assist the customer to repair the defective products until the problem is solved.
What is not covered?
- Product purchased from anyone other than a SISCO store or a SISCO authorized reseller.
- Expendable parts.
- Routine cleaning or normal cosmetic and mechanical wear.
- Damage from misuse, abuse or neglect.
- Damage from use of parts other than SISCO approved.
- Damage from use outside the product’s usage or storage parameters.
- Damage from use of parts not sold by SISCO.
- Damage from modification or incorporation into other products.
- Damage from repair or replacement of warranted parts by a service provider other than a SISCO authorized service provider.
- Damage caused by the application environment not meeting the product usage requirements and the failure to perform preventive maintenance.

