SISCO portable colorimeter measuring aperture 8mm, easy to operate, aesthetic design combined with ergonomic structure, high hardware configuration, available for automotive painting, textile, leather, and other color inspection.
3.5-inch touch screen
- 3.5-inch large capacitive touch screen with full-function touch control, making the Color Spectrophotometer more intuitive and user-friendly for precise color analysis.
- 15-degree screen upside-down display.
- Large storage space and high hardware configuration.

Software button for one-touch measurement
- The back of the body is designed with software buttons, the bracelet is easy to hold and achieves easy measurement.
- The component screw slot, fixed components, to achieve more The measurement of products in more shapes.
- Large 58mm integrating sphere for accurate measurement.
Application
The colorimeter can easily achieve accurate color transmission and can also be used as precision color testing equipment. It is widely used in plastic, electronics, paint, ink, textile and garment, printing and dyeing, printing paper, automotive, medical, cosmetic and food industries, research institutes, schools and laboratories.
| Model | SISCO-CM-NS800 |
| Illumination/Observation System | 45/0 method (45 ring-shaped illumination, vertical viewing), Comply with CIE No.15,GB/T 3978 |
| Integrating Sphere Size | φ58mm |
| Light Source | Combined LED sources |
| Errors Between Each Equipment | ≤0.20E*ab |
| Sensor | Silicon photodiode array |
| Wavelength Range | 400~700nm |
| Wavelength Interval | 10nm |
| Reflectance Range | 0-200% |
| Measuring Aperture | φ8mm |
| Color Space | CIE LAB,XYZ,Yxy,LCh,CIE LUV |
| Color difference Formula | ΔE*ab,ΔE*uv,ΔE*94,ΔE*cmc(2:1),ΔE*cmc(1:1),ΔE*00 |
| Other Chromaticity Data | WI(ASTM E313,CIE/ISO, AATCC, Hunter), YI(ASTM D1925,ASTM 313), TI(ASTM E313,CIE/ISO), Metamerism Index (Mt), Color Stain, Color Fastness |
| Observer | 2°/10° |
| Illuminant | D65,A,C,D50,D55,D75,F2,F6,F7,F8,F10,F11,F12 |
| Display Data | Spectral Value/Graph, Colorimetric Value, Color Difference Value/Graph, PASS/FAIL Result, Color Offset, Color Simulation |
| Measurement Time | 1.5s |
| Repeatability | Spectral Reflectance: standard deviation within 0.1%(400~700nm: within 0.2%) |
| Colorimetric Value | Standard deviation within Delta E*ab 0.04 (Measurement conditions: white calibration plate measured 30 times at 5 seconds intervals after white calibration was performed.) |
| Inter Instrument Agreement | Within Delta E*ab 0.2 (Average for 12 BCRA Series II color tiles) |
| Dimension | 90*77*230mm |
| Weight | 600g |
| Battery | Li-ion battery. 5000 times within 8 hours |
| Lamp Life | 5 years, more than 1.6 million measurements |
| Display Screen | USB/RS-232 |
| Interface | 1000 Standards, 15000 Samples |
| Data Memory | Data Memory |
| Operating Temperature | -20~50℃(-4~122°F) |
Note: The user can perform black-and-white calibration through the standard black-and-white box of the instrument and refer to the user manual to complete the calibration independently.
Color Spectrophotometer Accessories
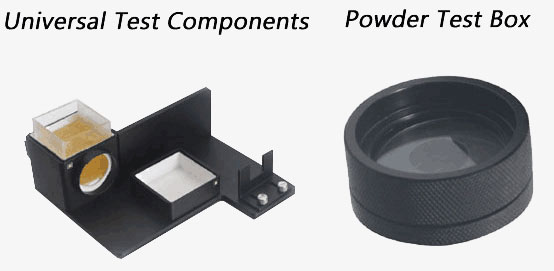
1. Universal Test Components
Features: Powerful accessory with high sensitivity which is used to measure liquid, paste and powder.
Dimension: L*W*H=190x190x85 mm
2. Powder Test Box
Features: Easy to use; Focus on measuring powder.
Dimension: ø50X25mm
Packing List
- 1 x Spectrophotometer
- 1 x Power adapter
- 1 x Lithium battery
- 1 x Manual
- 1 x CD (including management software)
- 1 x Data cable
- 1 x White correction tube
- 1 x Protection cap
- 1 x Wristband
- 1 x Universal test module (Optional match)
- 1 x Powder test box (Optional match)
Q1: How does a colorimeter work?
A1: Colorimeters rely on the concept of the Beer-Lambert law, which assumes that the absorbance of a substance is proportional to its concentration. For example, the higher the concentration of a solution, the higher the absorbance reading. Many chemical and biological experiments are based on this concept. To obtain a Beer's law curve, several standards (solutions of known concentration) are prepared and their absorbance values are determined using a colorimeter.
Q2: What is colorimetry?
A2: A colorimeter is a device that measures light absorbance (how much light is absorbed) and light transmission (how much light passes through) in a liquid by analyzing the intensity of the color.
Q3: What are the limitations of a colormeter?
A3: One limitation of chemical colormeters is that differences in certain substances may lead to inaccurate test results. According to Global Water Instrumentation, because these differences are different for each substance, a chemical colorimeter alone is not a completely foolproof testing device.
Tips: How does the portable colorimeter detect the color of a pill coating?
A conventional colorimeter is a visual system that simulates the human eye. Using the simulated integral optical system inside the instrument, the tri-stimulus values X, Y, and Z of the sample are integrated and measured, and then parameters such as the chromaticity coordinates of the sample are calculated. The color measurement accuracy of this colorimeter is relatively low. Some products cannot even give the absolute value of the color coordinate space (L, a, b values). Since there is no absolute value data of color, it is impossible to exchange data with others. You cannot create and manage your own color standard database.
A portable colorimeter uses the principle of spectrophotometry to compare the light energy reflected (transmitted) by the sample with the light energy reflected (transmitted) by the standard under the same conditions to obtain the spectral reflectance of the sample at each wavelength, and then uses the standard observer and standard light source provided by CIE to calculate the trimodal values X, Y, and Z according to the following formula, and then calculates them according to the CIEYxy, CIELab, and other formulas to calculate the degree coordinates x. y, CIELAB color parameters, etc. The measured color difference results are more accurate, and a color standard database can be established. However, the surface of the drug is irregular and the surface is not flat. Therefore, when choosing a spectrophotometer, we also need to pay attention to different measurement and observation methods.
The 0/45 degree can only be used to measure smooth surfaces and cannot be used for computer color matching. d/8 degree integrating sphere can be used to measure various surfaces and can be used for computer color matching. Also, consider whether you want to eliminate specular reflections and the measurement modes that include specular reflections when making your selection. If both modes are available, it is useful for measuring smooth surfaces where its color can clearly reflect the surface.
Thank you for buying industrial test and measurement equipment on SISCO.com, all products sold by SISCO and the partner cover a 12 months warranty, effective from the date of receiving the products.
What is covered?
SISCO is responsible for providing free spare parts, and free technical support to assist the customer to repair the defective products until the problem is solved.
What is not covered?
- Product purchased from anyone other than a SISCO store or a SISCO authorized reseller.
- Expendable parts.
- Routine cleaning or normal cosmetic and mechanical wear.
- Damage from misuse, abuse or neglect.
- Damage from use of parts other than SISCO approved.
- Damage from use outside the product’s usage or storage parameters.
- Damage from use of parts not sold by SISCO.
- Damage from modification or incorporation into other products.
- Damage from repair or replacement of warranted parts by a service provider other than a SISCO authorized service provider.
- Damage caused by the application environment not meeting the product usage requirements and the failure to perform preventive maintenance.