Conductivity meters play a crucial role in various scientific and industrial applications by providing a quick and accurate measure of a solution's ability to conduct electricity. In this blog post, we will delve into the working principles of conductivity meters, exploring the electrochemical methods employed to measure the conductivity of electrolyte solutions.
Conductivity Meter Working Principle
The method of measuring the conductance of the solution to be measured is called conductivity analysis. Conductance is the reciprocal of resistance, so the measurement of conductance value is actually converted by measuring the resistance value. That is to say, the measurement method of conductance should be the same as the measurement method of resistance. However, during the measurement of solution conductance, when current passes through the electrode, ions will discharge on the electrode, causing polarization and causing errors. Therefore, an alternating current with a sufficiently high frequency must be used when measuring conductance to prevent the generation of electrolytic products. In addition, the electrodes used are plated with platinum black to reduce overpotential and improve the accuracy of measurement results. The quantity we are more interested in is conductivity.
Conductivity is a numerical representation of a solution's ability to conduct electrical current. The conductivity of water has a certain relationship with the amount of inorganic acids, alkalis, and salts it contains. When their concentrations are low, the conductivity increases as the concentration increases. Therefore, this indicator is often used to estimate the total number of ions in water. concentration or salt content.

Conductivity and water hardness
The conductivity of an aqueous solution is directly proportional to the concentration of dissolved solids, and the higher the concentration of solids, the greater the conductivity. The relationship between conductivity and dissolved solids concentration is approximately expressed as: 1.4μS/cm=1ppm or 2μS/cm=1ppm (CaCO3 per million units). The total hardness value of water can be obtained indirectly by using a conductivity meter or a total dissolved solids meter. As mentioned above, for the convenience of approximate conversion, 1μs/cm conductivity = 0.5ppm hardness
Note
- Use conductivity to indirectly measure water hardness, with a theoretical error of about 20-30ppm.
- The conductivity of the solution determines the movement of molecules, and temperature affects the movement of molecules. In order to compare the measurement results, the test temperature is generally set at 20°C or 25°C.
- Reagent detection can be used to obtain a more accurate water hardness value.
Soft water and hard water
Water is divided into soft water and hard water. Water that does not contain or contains a small amount of calcium and magnesium ions is called soft water, and vice versa is called hard water. The hardness component of water, if caused by sodium bicarbonate or magnesium bicarbonate, is temporary hard water (boiling temporarily hard water causes the decomposition of sodium bicarbonate to precipitate the insoluble carbonate generated, and the water changes from hard water to soft water) ; If it is caused by sulfates or chlorides containing calcium and magnesium, it is permanent hard water. According to the total hardness value of water, it is roughly divided into soft water with a total hardness of 0-30ppm, hard water with a total hardness of 60ppm and above, high-quality drinking water not exceeding 25ppm, and high-quality soft water with a total hardness of less than 10ppm. In natural water, unpolluted rainwater and snow water away from cities are soft water; spring water, stream water, river water, and reservoir water are mostly temporarily hard water, and some groundwater is high hardness water.
Regarding the conversion of common units for water hardness conversion
1mmol/L (1/2Ca2+, 1/2Mg2+)=50 ppm (calculated as CaC03)
1mmol/L(1/2Ca2+, 1/2Mg2+)=2.92grain/gallon
lgrain/gallon =17.lppm (calculated as CaC03)
1m3=264gallon (US)=22Oganon(UK)
lkg=2.2pounds
1ppm=1mg/L
TDS (Total Dissolved Solids) is a measure of the total content of all ions in water, usually expressed in ppm.
In pure water manufacturing, conductivity can also be used to express indirectly as TDS.
The conductivity of a solution is equal to the sum of the conductivities of the various ions in the solution.
The empirical formula is: Halving the conductivity in microsiemens is approximately equal to TDS (ppm)
Sometimes TDS is also expressed by other salts, such as CaCO3 (the coefficient is 0.66)
The conversion coefficient between TDS and conductivity can be adjusted between 0.4-1.0 to correspond to different types of electrolyte solutions.
Conductivity
Conductivity is the ability of a substance to carry electric current, as opposed to resistance, and its unit is cm.
The working principle of the conductivity meter is shown in the figure.
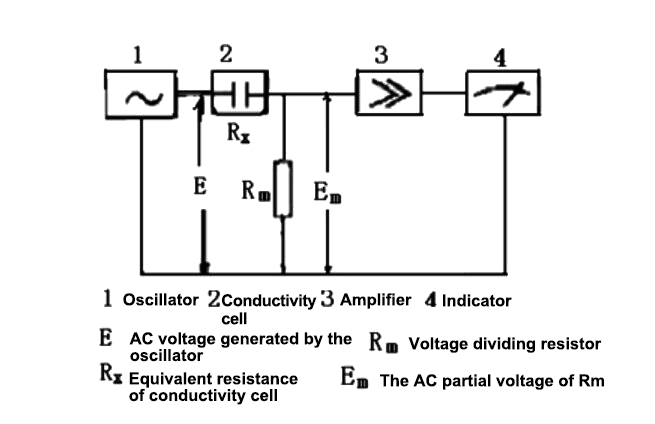
From Ohm's law we know:
Em = ERm / (Rm+Rx) = ERm÷( Rm+Kcell/κ)
Kcell is the conductivity cell constant, and κ is the conductivity. When E, Rm and Kcell are all constants, changes in the conductivity κ will inevitably cause changes in Em. Therefore, measuring the size of Em also measures the value of the conductivity of the solution. . The Em value is sent to the AC amplifier for amplification, and then the signal is rectified to obtain the DC signal output that drives the meter, and the meter directly reads the conductivity.
The conductance (G) of a sample is the reciprocal of the resistance (R). When two electrodes (usually platinum electrodes or platinum black electrodes) are inserted into the solution, the resistance R between the two electrodes can be measured. According to Ohm's law, when the temperature is constant, this resistance value is proportional to the electrode spacing L (cm). It is inversely proportional to the electrode cross-sectional area A (cm2), and the formula is as follows:
R = ρ* (L/A)
G = 1/R = 1/ρ* (A/L) = κ*(1/J)
Among them, ρ is the resistivity, κ is the conductivity, κ = 1/ρ; J = L/A is the electrode constant.
The conductivity of an electrolyte solution is the conductivity of 1cm3 solution between two parallel electrodes 1cm apart. When the electrode constant is known, the conductivity can be obtained by measuring the conductance or resistance of the solution.
Water hardness
Water hardness refers to the concentration of calcium and magnesium ions in the water. The unit of hardness is ppm. 1ppm represents 1 mg/L of calcium carbonate in the water.
Conductance (G) is the reciprocal of resistance (R). Therefore, when two electrodes (usually platinum electrodes or platinum black electrodes) are inserted into the solution, the resistance R between the two electrodes can be measured. According to Ohm's law, when the temperature is constant, the resistance value is directly proportional to the electrode spacing L (cm) and inversely proportional to the cross-sectional area A (cm2) of the electrode, that is, R = ρ × (L/A); where ρ is the resistivity, which is the length 1cm, the cross-sectional area is 1cm2. The resistance of the conductor is determined by the nature of the material.
According to the above formula, the conductance (G) of the conductor can be expressed as the following formula: G =1/R= (1/ρ) × (A/L) = K × (1/J); among them, K = 1/ρ is called is the conductivity, J = L/A is called the electrode constant; the conductivity of the electrolyte solution refers to the conductance when two parallel electrodes 1 cm apart are filled with 1 cm3 of solution. It can be seen from the above formula that when the electrode constant (J) is known and the solution resistance (R) or conductance (G) is measured, the conductivity can be calculated.
The conductivity meter is a reliable laboratory instrument, and if you want to learn more about it, stop by sisco.

