Spectrophotometers are instruments used to measure the concentration of substances, and their basic principle involves determining the concentration of a substance based on its absorbance properties. The measurement principle is founded on Beer-Lambert's Law, which states that the absorbance of light by a substance is directly proportional to the concentration of the substance and inversely proportional to the path length of the light. Spectrophotometers achieve this by breaking down visible or ultraviolet light into different wavelengths and then measuring the intensity of light absorbed by the sample at different wavelengths to determine the substance's concentration. In this article, Sisco will briefly introduce spectrophotometer to you, including its components, working principle, classifications, usage process and applications etc., to help you have a better understanding of it.
Components of Spectrophotometer
Components of a spectrophotometer include a light source, a grating, a sample chamber, and a detector. The light source generates visible or ultraviolet light, the grating disperses the light into different wavelengths, the sample chamber holds the sample, and the detector measures the intensity of the light absorbed by the sample. The sample chamber is typically a transparent glass or quartz chamber, and the sample is placed within it. The detector is commonly a photodiode or a photomultiplier tube used to measure the intensity of the absorbed light.
Working Principle of Spectrophotometer
The operating principle of a spectrophotometer is based on spectrophotometry, where the absorbance of light by the substance under examination at specific wavelengths or within a certain wavelength range is measured for qualitative and quantitative analysis.
Different types of spectrophotometers share a similar basic principle, utilizing a light source capable of producing multiple wavelengths. Through a series of optical components, specific wavelengths of light are generated. After passing through the sample, some of the light is absorbed. By measuring the absorbance of the sample and performing subsequent calculations, the concentration of the sample can be determined. The absorbance value is directly proportional to the concentration of the sample.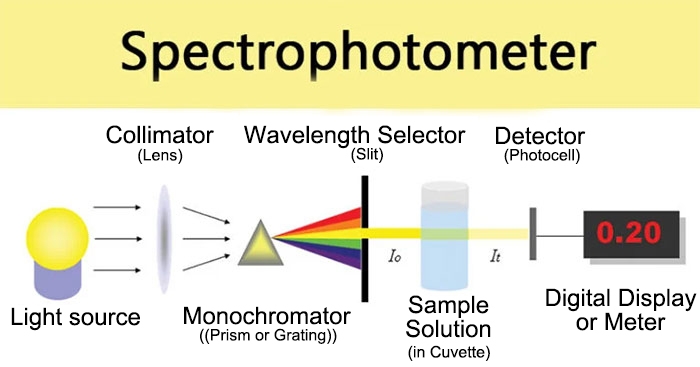
Classification of Spectrophotometers
- According to wavelength and application areas:
- Visible Spectrophotometer: Measures the wavelength range in the visible light region, typically 400 to 760 nm.
- UV Spectrophotometer: Measures the wavelength range in the ultraviolet light region, typically 200 to 400 nm.
- IR Spectrophotometer: Measures the wavelength range in the infrared light region, typically greater than 760 nm.
- Fluorescence Spectrophotometer: Used for scanning the fluorescence spectrum emitted by liquid-phase fluorescent markers.
- Atomic Absorption Spectrophotometer: The light source emits characteristic spectral radiation of the substance being measured. The ground-state atoms of the test element in the sample vapor, after passing through the atomizer, absorb the radiation. The concentration of the test element is determined by measuring the absorbed radiation.
- According to the degree of automation: Can be classified as manual, semi-automatic, and automatic spectrophotometers.
- According to software: With scanning capabilities or without scanning capabilities.
The Usage Process of Spectrophotometers
The typical usage process of a spectrophotometer includes calibration, sample preparation, setting instrument parameters, measure the intensity of absorbed light by the sample, and calculating the concentration of the sample.
During calibration, known concentrations of standard solutions are used to adjust the instrument settings to ensure accuracy and precision. Sample preparation involves dissolving the sample in an appropriate solvent and placing it in the sample chamber. Setting instrument parameters includes selecting the appropriate light source, wavelength range, and detector sensitivity.
Measuring the intensity of absorbed light by the sample involves comparing the sample in the sample chamber with a standard solution to determine the sample's concentration. Finally, the concentration of the sample is calculated based on Beer-Lambert's Law.
Applications of Spectrophotometers
- Visible Spectrophotometer:
Used to measure the absorbance of the test substance to visible light (400-760 nm) and perform quantitative analysis. It can be employed, for example, to measure bacterial cell density at 600 nm. - UV-Visible Spectrophotometer:
UV-Visible spectrophotometers are utilized to measure the absorbance of the test substance to visible or ultraviolet light (200-760 nm) and perform quantitative analysis. They can be used for determining the concentrations of nucleic acids and proteins, as well as measuring bacterial cell density. UV-Visible spectrophotometers can be classified into single-beam, pseudo-double-beam, and double-beam instruments, each serving different purposes.
- Single-beam: Suitable for measuring absorbance or transmittance at a given wavelength, generally incapable of full-spectrum scanning, requiring high stability of the light source and detector.
- Pseudo-double-beam: Also known as ratio double-beam, it splits light from the same monochromator into two beams. While advantageous for monitoring errors caused by changes in the light source, it does not eliminate the impact of the reference.
- Double-beam: Automatically records and enables rapid full-spectrum scanning. It can eliminate factors such as unstable light sources and detector sensitivity changes, making it particularly suitable for structural analysis. However, these instruments are complex and relatively expensive.

- IR Spectrophotometer:
Infrared spectrophotometers are used to study infrared spectra, typically referring to infrared spectra greater than 760 nm. This method is commonly used for the analysis of organic compounds in various states (gas, liquid, solid). - Fluorescence Spectrophotometer:
Used for scanning the fluorescence spectra emitted by liquid-phase fluorescent markers. It finds applications in research, chemical engineering, pharmaceuticals, biochemistry, environmental protection, clinical testing, food inspection, educational experiments, and more. - Absorbance Meter:
This method is mainly applicable to the analysis of trace and ultratrace components in samples. It is a powerful tool for material analysis and quality control departments to analyze trace amounts of metals (semimetals).
Spectrophotometers are precise instruments for accurately measuring substance concentrations. Their principle is based on Beer-Lambert's Law, involving the measurement of the intensity of absorbed light at different wavelengths after breaking down visible or ultraviolet light. Spectrophotometers find widespread applications in fields such as biochemistry, environmental monitoring, pharmaceutical research, and more.

